INTEGRATED PARAMAGNETIC RESONANCE AND OPTICAL MULTI-DIMENSIONAL BIO-SPECTROSCOPY
iPRO-MD-BioSpec @ HUN-REN Biological Research Centre, Szeged
Coordinator: Tibor Páli, Membrane Biophysics Research Group, Institute of Biophysics
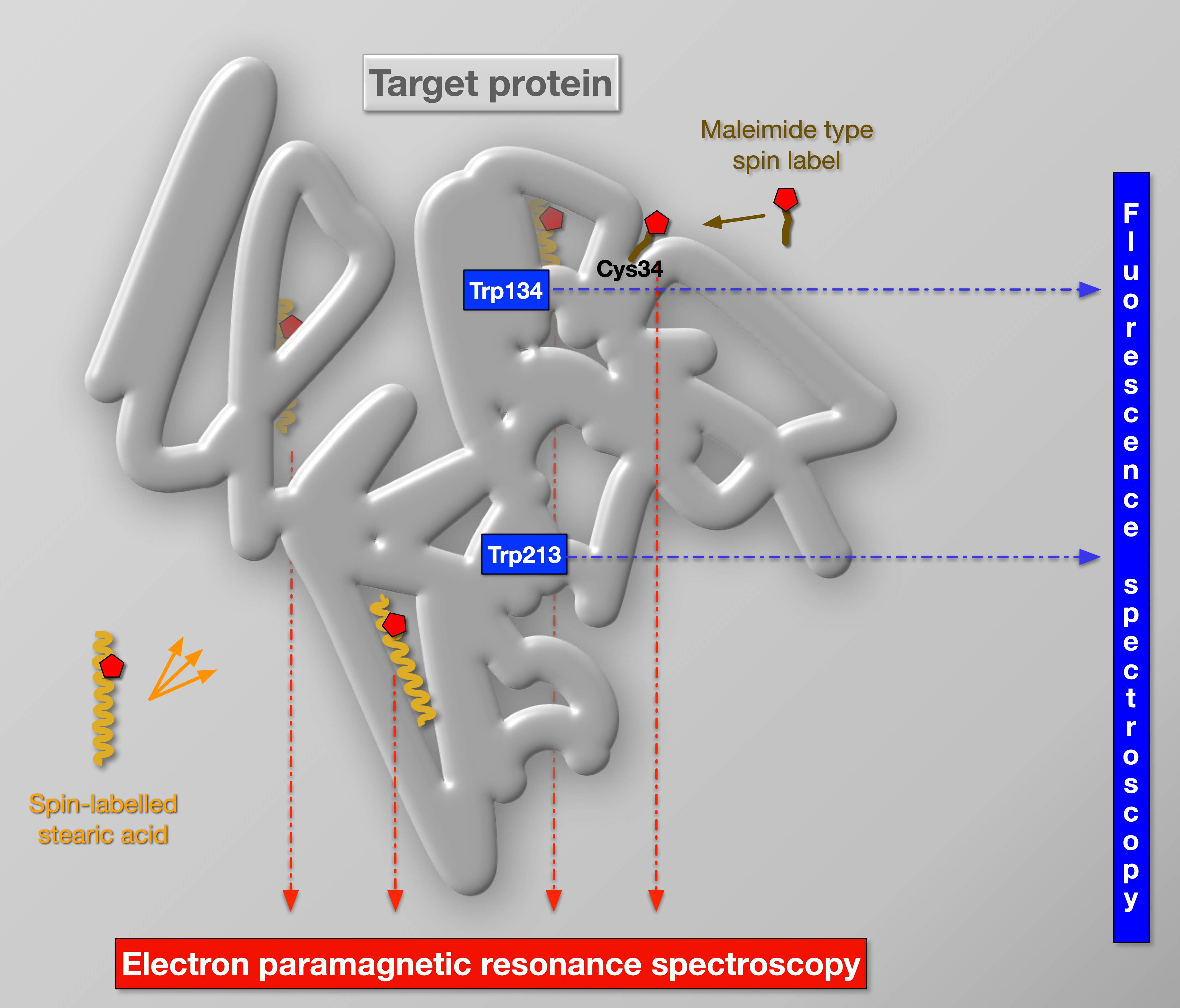
Concept and vision
Spectroscopic techniques are key to Biophysics because of the unique information they can provide about biological systems. Indeed, state-of-the-art spectroscopy yields unprecedented spatial (down to atomic) and temporal (down to femtosecond) details even in native systems. Our concept and vision had been evolving as follows: (i) The combination of complementary techniques had long been employed by us to multiply the information on biomolecular structure and dynamics gained. The experimental data are consistently interpreted in detailed molecular and physical models and related to the biological function. (ii) Whenever it is possible, we measure the same sample with complementary spectroscopic techniques, because this approach brings the quality and consistency of data to the next level. The core of our inter-connected spectroscopy infrastructure are EPR and fluorescence. Efficient integration is possible with optical fibres and flow systems (for liquid samples) – we have already prototyped this between the EPR and the fluorescence spectrometer (with an optical fibre) and between the EPR, a spectrophotometer and a refractive index measuring device (with a flow-through system). (iii) Once integrating multi-modal (paramagnetic resonance and several optical) spectroscopies, spectra can be recorded in a systematic way and a wealth of data is obtained in the form of a multi-dimensional matrix. The high information content of this data can be analysed with artificial intelligence (AI) to fingerprint certain relevant physiological states of any biological system. (iv) We are also working on detecting electron transfer interactions between paramagnetic centres (spin labels, free radicals, metal complexes) and fluorescent molecular groups (native or dyes) in EPR-fluorescence setup interconnected with fibre optics. In a worldwide unparalleled way, this will enable not only simultaneous EPR and fluorescence measurements on the same sample (in the EPR resonator), but also a direct detection of interactions between fluorescent molecular groups and free radicals, spin labels and paramagnetic metal centres, opening up a completely unexploited field, which holds novel opportunities for determining structural and dynamic data in biological samples.

Research Instruments of iPRO-MD-BioSpec
- Our primary and highest value (~1 million EUR) instrument is a Bruker ELEXSYS E580 X-band CW and pulsed-, FT- electron paramagnetic resonance (EPR) spectrometer, installed in 2018. EPR is the most direct and most powerful method to study paramagnetic molecules. It is an expensive and difficult technique but it finds strong multi- and interdisciplinary applications solving relevant problems in physics, chemistry, biology and even medicine, because different classes of paramagnetic molecules (metal complexes, free radicals, spin labels) are targets and subjects of cutting edge basic research in these disciplines. In our hands, EPR is also entering more and more into applied research. EPR has been one of the most productive spectroscopic methods of BRC since the 70’s (contributing to more than 70 research papers). Our new E580 spectrometer is the highest grade and best equipped EPR spectrometer in Central- and East Europe: it has four different resonators, arbitrary waveform generator, electron-nuclear double resonance unit, and helium and nitrogen temperature controllers (covering 5-500K). The instrument has all the state-of-the-art CW and pulsed/FT measurements modes. Our E580 is mainly used for site-specific spin labelling and spin-trapping (free radical detection) but we also do EPR experiments on metal complex and metal proteins. (Maintained by the Membrane Biophysics Research Group.)
- The Horiba (Jobin-Ivon) Fluorolog 3 (FL3-222) modular fluorescence spectrometer is equipped with time-correlated single photon counting (TCSPC), 96-plate reader, front-face, angled fluorescence detection (for solid samples) and a thermostat. The instrument has been upgraded recently to the "T-arm" configuration, that means two emission units, with independent monochromators, polarisers and detectors. Our FL-322 fluorescence spectrometer is among the most used instruments in the BRC for routine experiments, serving numerous independent projects (and ~20 publications in the past 5 years). (Maintained by the Membrane Biophysics Research Group.)
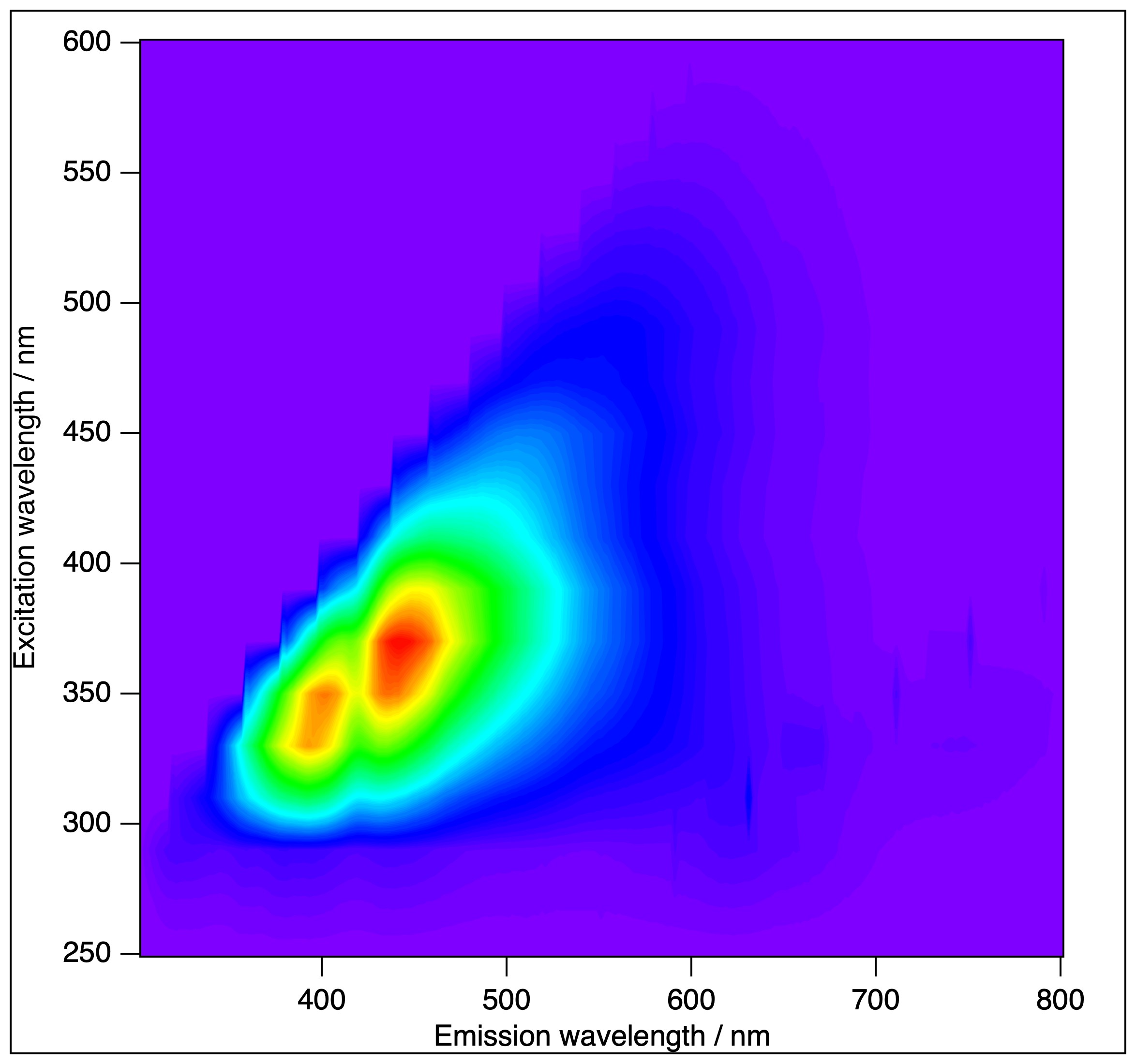
- The Femtobiology laboratory is a laser driven fluorescence setup capable of detecting fluorescence kinetics in the 100 fs – 10 ns time window and in the complete visible spectral range, aided with sophisticated custom software to analyse fluorescence kinetics data. The third harmonic generation of the pump laser of the time resolved fluorescence spectroscopy setup brought new classes of molecules as potential targets of research. It is the only setup in Hungary combining fluorescence up-conversion and time correlated single photon counting, giving a unique time resolution and measurement interval. (Maintained by the Protein Biophysics Research Group.) A permanent workstation is also being developed at the ELI ALPS (Szeged) for studying ultrafast processes on biological systems utilising light energy. The measuring techniques to be applied are founded partially on the expansion of the most modern technology of femtosecond multidimensional electronic spectroscopy, and partially on the improvement of the methods we developed for the detection of low-intensity light-induced coherent THz radiation. On concluding with the complete implementation, the workstation will be publicly available for users.
- Kinetic optical spectroscopy laboratory. We develop and apply general chemometric methods for the analysis of spectral data matrices in order to determine the underlying physical phenomena of biological processes and systems. In addition, we study the possibilities of the integration of „colored” proteins in biophotonic and in bioelectronic applications. Our main experimental setup consists of a tunable Nd-Yag + OPO pulse laser to provide < 10 ns actinic pulses and a continuous white measuring light source in the entire visible range. Time resolved spectra of the excited sample can be measured at delay times from <100ns upwards. High time resolution single wavelength kinetics can also be measured in the same experimental arrangement. (Maintained by the Protein Biophysics Research Group.)
- Also available: Microcal VP-DSC high-sensitivity difference scanning calorimeter and Bruker Vertex 70 Fourier-transform infrared spectrometer equipped with polarised attenuated total internal reflection (PATIR) option and a thermostat. (Maintained by the Membrane Biophysics Research Group.)
iPRO-MD-BioSpec for basic research
Our own focus is on biomembranes and proteins, and the specific research fields are as follows: (i) Molecular biophysics of the structure-function relationship and working mechanism of selected ion-transporting membrane proteins. (ii) Combining spectroscopic data with bioinformatics, artificial intelligence (AI) and molecular mechanics (MM) and -dynamics (MD) to build atomistic and physical models of membranes and proteins and understand their mechanism of action. (iii) Protein-lipid interactions and membrane reorganisations in photosynthetic, membrane fusion and lipid-based drug delivery processes. (iv) For proteins with chromophoric prostetic groups spectroscopic and kinetic spectroscopic studies in the visible-UV range provide essential information about the protein function. (v) In addition, relying on this unique molecular biophysics suite of methods, we also participate in numerous collaborative projects related to photosynthesis, agriculture and environment protection, such as studying free radicals, native biomembranes, membrane-associated proteins, drug delivery processes.
iPRO-MD-BioSpec for applied research
Thank to our unique research infrastructure, we are involved in several national and international R&D projects aimed at protecting the environment and improving the quality of life. For instance, we routinely use EPR for characterising intermediate and final food products regarding their free radical content. In Hungarian-Egyptian, and BRC-University of Szeged collaborations we aid the development of wastewater cleaning technology with spectroscopic characterisation of selective membrane-filters for purification of waste water from organic contaminates. In a new, medically oriented joint project with the Institute of Biophysics of Pécs University (Hungary) we are seeking for the spectroscopic fingerprints in the plasma membrane of erythrocytes from patients with diabetes mellitus.
We strongly believe in the synergistic impacts and mutual benefits of collaborations in research. Our instruments serve numerous projects in the BRC and outside (including international collaborations). Please contact us for discussions about what iPRO-MD-BioSpec can bring to your project (be it a basic or applied research in any fields of biology, medicine, chemistry, physics and material sciences). Independent access for routine fluorescence and optical absorption experiments is also possible, and it is subject to a signed agreement and meeting certain basic conditions (contact us for details).
Selected recent publications
*Páli, T., and Kóta, Z. (2019). Studying Lipid-Protein Interactions with Electron Paramagnetic Resonance Spectroscopy of Spin-Labeled Lipids. Methods Molecular Biololgy 2019, 2013, 529-561.
Krekic, S., Zakar, T., Gombos, Z., Valkai, S., Mero, M., Zimányi, L., *Heiner, Z. and *Dér, A. (2020). Non-linear optical investigation of microbial chromoproteins. Frontiers in Plant Science 11:547818. DOI
Lingvay, M., Akhtar, P., Sebők-Nagy, K., Páli, T., and *Lambrev, P. H. (2020). Photobleaching of Chlorophyll in Light-Harvesting Complex II Increases in Lipid Environment. Frontiers in Plant Science 11, 849. DOI
*Zimányi, L., Thekkan, S., Eckert, B., Condren, A.R., Dmitrenko,O., Kuhn, L.R., Alabugin, I.V. and *Saltiel, J. (2020). Determination of the pKa values of trans-Resveratrol, a Triphenolic Stilbene, by Singular Value Decomposition. Comparison with Theory. J. Phys. Chem. A 124(31):6294-6302. DOI
Khoroshyy, P., Tenger, K., Chertkova, R.V., Bocharova, O.V., Kirpichnikov, M.P., Borovok, N., Groma, G.I., Dolgikh, D.A., Kotlyar, A.B. and *Zimányi, L. (2021) Kinetics and energetics of intramolecular electron transfer in single-point labeled TUPS-cytochrome c derivatives. Molecules 26(22):6976. DOI
Rehman, A. U., Bashir, F., Ayaydin, F., Kóta, Z., Páli, T., and *Vass, I. (2021). Proline is a quencher of singlet oxygen and superoxide both in in vitro systems and isolated thylakoids. Physiologia Plantarum 172, 7-18. DOI
Zimányi, L., Sipos, Á., Sarlós, F., Nagypál, R. and *Groma, G. (2021) Machine learning-based model selection and parameter estimation from kinetic data of complex first-order reaction systems. PLOS One 16(8): e0255675. DOI
Bérczi, A., Márton, Z., Laskay, K., Tóth, A., Rákhely, G., Duzs, Á., Sebők-Nagy, K., Páli, T., and *Zimányi, L. (2023). Spectral and Redox Properties of a Recombinant Mouse Cytochrome b561 Protein Suggest Transmembrane Electron Transfer Function. Molecules 28(5), 2261. DOI
Misra, R., Das, I., Dér, A., Steinbach, G., Shim, J-G., Busse, W., Jung, K-H., *Zimányi, L. and *Sheves, M. (2023) Impact of protein-chromophore interaction on the retinal excited state and photocycle of Gloeobacter rhodopsin: role of conserved tryptophan residues. Chemical Science 14:9951. DOI
Sebők-Nagy, K., Blastyák, A., Juhász, G., and *Páli, T. (2023). Reversible binding of divalent cations to Ductin protein assemblies-A putative new regulatory mechanism of membrane traffic processes. Frontiers in Molecular Biosciences 10, 1195010. DOI
Sebők-Nagy, K., Kóta, Z., Kincses, A., Fazekas, Á. F., Dér, A., László, Z., and *Páli, T. (2023). Spin-Label Electron Paramagnetic Resonance Spectroscopy Reveals Effects of Wastewater Filter Membrane Coated with Titanium Dioxide Nanoparticles on Bovine Serum Albumin. Molecules 28, 6750. DOI
Laczkó-Dobos, H., Bhattacharjee, A., Maddali, A.K., Kincses, A., Abuammar, H., Sebők-Nagy, K., Páli, T., Dér, A., Hegedűs, T., Csordás, G. and *Juhász, G. (2024). PI(4)P promotes Syntaxin 17 recruitment to autophagosomes for lysosomal fusion. Autophagy. DOI.
*Páli, T., Feniouk, B. and Wilkens, S. (2024). Editorial: Functions, Working Mechanisms, and Regulation of Rotary ATPases and Ductin Proteins. Frontiers in Molecular Biosciences 11, 1399421. DOI
*Emam, H. E., Koto , T., Sebők-Nagy, K., El-Shahat, M., Abdel-Gawad, H., *Páli, T. and *Abdelhameed, R. (2024) Synthesis, Spectroscopic Study and Carbofuran Adsorption of Mixed Metal (Co, Cu)@Ca-BTC Frameworks aimed at Wastewater Cleaning. Separation and Purification Technology DOI.
